Summary:
A failure of the flow sensor in several GE Engstrom Ventilators was identified. To remedy this failure, these sensors have been replaced in all of the affected ventilators. Additionally, to prevent future failures, a new annual replacement cycle has been put into place.
Details:
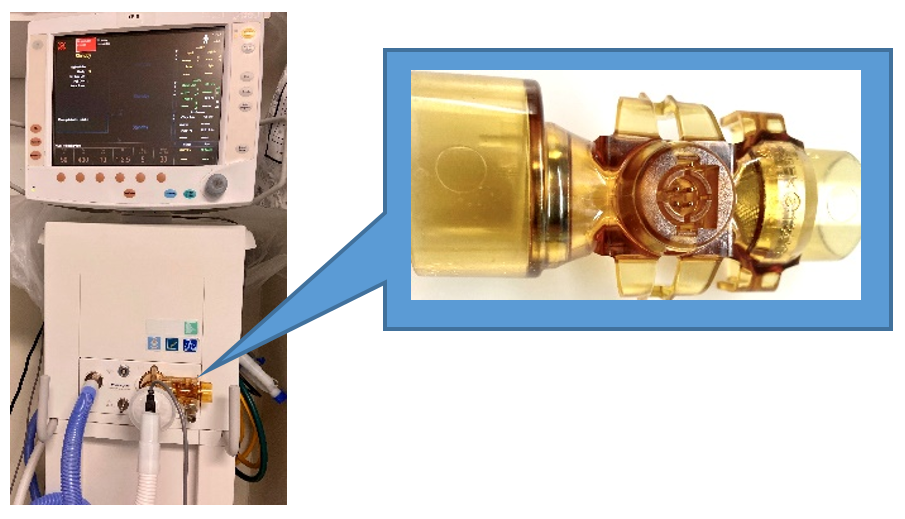
Respiratory Therapists and Critical Care identified a critical issue with a type of ventilator used at Cooper (GE Engstrom). When one failed, the care team took action immediately. Not only did they immediately protect the patient and prevent harm, they also placed an EARS event and escalated their concerns. While investigating the incident, other events occurred. The Respiratory team, in conjunction with Critical Care, went that extra mile to place additional EARS events as well as involving Clinical Engineering and Patient Safety.
By relying on the expertise of our frontline staff, we were able to identify the root cause of the failure; a specific sensor in the ventilator, a flow sensor in the expiratory valve. Clinical Engineering used their expertise to identify all the ventilators that had this sensor. They then quickly replaced all of the affected machines. Additionally, Respiratory and Clinical Engineering went further. They investigated the sensor and discovered that the failure was likely due to the repeated reprocessing of these accessory ventilator components. They took their plan to the next level. They replaced all of the sensors AND created a new annual replacement schedule to prevent the conditions that lead to the failure in the first place.
Kudos to all those involve in identifying, investigating, and correcting this issue!
Please e-mail any concerns, questions, or clarifications to kirchhoff-michael@cooperhealth.edu.