When Cook Medical recently received FDA approval for its Zenith® Dissection Endovascular System, it was a major milestone for Cooper—which served as the flagship institution for the clinical trial that led to the device’s clearance.
“This is the first and only “disease specific” system of its kind that provides a less invasive alternative to open surgery for repair of complicated type B dissections of the descending thoracic aorta,” says Joseph V. Lombardi, MD, FACS, Head, Division of Vascular and Endovascular Surgery and Director of the Cooper Aortic Center, who served as the study’s global principal investigator.
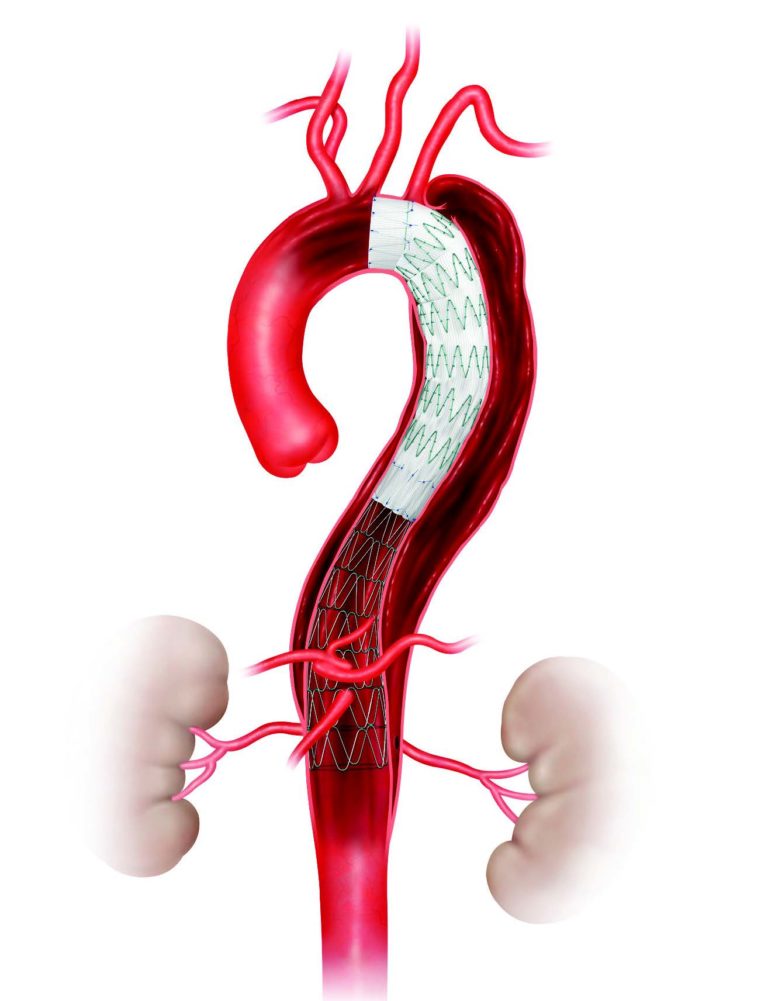
Zenith Dissection Device (permission for use granted by Cook Medical, Bloomington, Indiana)
Aortic dissection is a tear that occurs between the innermost and middle layers of the aorta. When the inner layer of the aorta tears, blood flows through the tear, causing the inner and middle layers of the aorta to separate (dissect). Type B dissection involves a tear in the lower (descending) aorta, while Type A dissection involves a tear in the upper (ascending) aorta.
Although uncommon, most aortic dissections occur because high blood pressure causes the artery’s wall to deteriorate. Aortic dissection symptoms may be similar to those of other heart problems and typically include sudden, excruciating pain, most frequently across the chest but also in the back between the shoulder blades.
The Zenith system consists of a proximal stent graft component and a distal bare stent component, which are delivered to the dissection site via catheter. Instead of making a large incision in the chest, the physician, without making an incision, enters the femoral artery near each hip to insert the device, then guides the device into place in the aorta.
Once in place, the device helps to prevent the aorta from bursting and can reestablish vital blood supply to other areas of the body. Before it was approved by the FDA, the device underwent two multiyear clinical trials with more than 160 patients in ten centers nationwide between December 2007 and August 2014.
“As the lead site for the clinical trial, Cooper has already acquired extensive expertise with this new system,” Dr. Lombardi notes. “So we’re able to offer it to appropriate South Jersey patients immediately.”
“Its availability also accelerates our goal to provide the region’s physicians with an array of durable, disease-specific treatment options that fit each patient’s unique anatomy and disease state,” he adds.
The leading-edge treatment options now available at the Cooper Aortic Center include interventions for complex aortic repair, abdominal aortic aneurysms (AAAs), arch aneurysms, thoracic aortic aneurysms (TAAs), thoracic and aortic dissections (type A and type B), and iliac aneurysms.
“As a clinician and researcher, I found it professionally rewarding to lead a multinational team to develop this new, minimally invasive treatment option,” said Dr. Lombardi. “Advances like this give the medical community, and ultimately our patients, more options for a successful outcome.” Dr. Lombardi and a team performed the first commercial procedure with this device in the United States in March 2019.
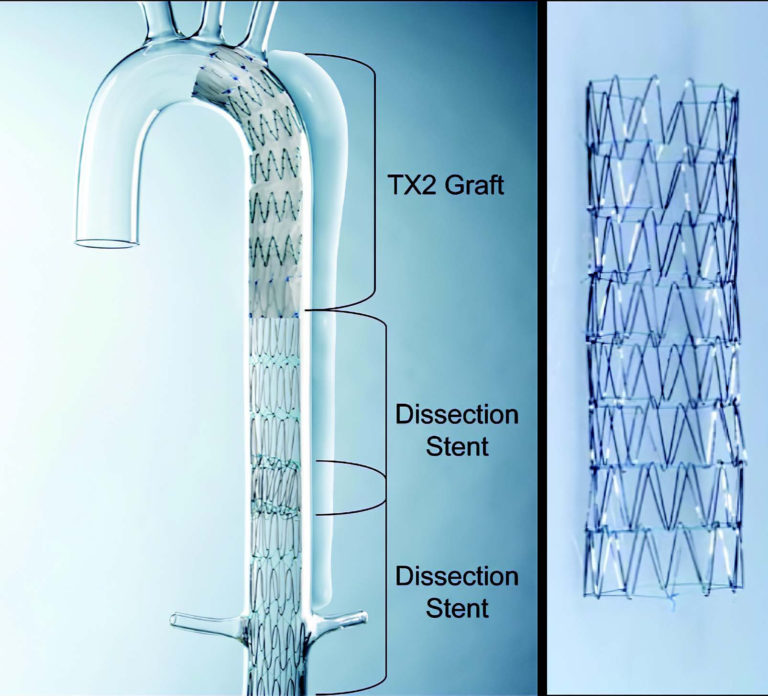
Illustration of the Zenith Dissection Endovascular System, comprising the Zenith TX2 thoracic aortic aneurysm Endovascular Graft with Pro-Form and the Zenith Dissection Endovascular Stent. The left panel shows one proximal TX2 endovascular graft and two distal dissection stents deployed in a model of Type B thoracic aortic dissection. The right panel shows a closer image of the bare metal dissection stent, which consists of multiple (four, six, or eight) self-expanding stainless steel z-stent segments sewn end-to-end with braided polyester suture.
Cooper’s Aortic Center has a team of seven vascular, endovascular, and cardiothoracic surgeons who specialize in the care and treatment of aortic disease—the only team of its kind in southern New Jersey. They perform more than 300 minimally invasive and open procedures a year. All are performed in a specially designed hybrid operating room that integrates sophisticated imaging and anesthesia with cutting-edge surgical technology.
These vascular and cardiothoracic surgeons are leaders in the endovascular repair of aortic disease and are renowned for their ability to perform complex open surgical procedures as well. They work closely with patients to identify the most appropriate treatment options for each patient’s disease and lifestyle.
Joseph V. Lombardi, MD, FACS, Head, Division of Vascular and Endovascular Surgery and Director of the Cooper Aortic Center.
