Cooper is the first and, to date, only hospital in New Jersey to offer bronchoscopic lung volume reduction (BLVR) therapy for patients with severe COPD/emphysema and hyperinflation. This minimally invasive procedure has been shown to help appropriately selected patients to experience less dyspnea, be more active and energetic, and enjoy a significantly improved quality of life compared with patients who receive medical management, the current standard of care.

Wissam Abouzgheib, MD, Division Head, Interventional Pulmonology
“Until the availability of these implanted endobronchial valves, all we could do for most patients with severe COPD/ emphysema was give them inhalers, start them on home oxygen, and refer them for lung transplantation,” says Wissam Abouzgheib, MD, Division Head, Interventional Pulmonology.
“Or, in selected patients with upper lobe-predominant emphysema and a low baseline exercise capacity, we could perform lung volume reduction surgery in which the surgeon would remove the upper lobes of both lungs,” he continues. “That’s a very invasive procedure, and these patients are compromised to begin with, so open surgery was associated with high risk.”
With BLVR, however, tiny valves are placed in the airways to occlude the most diseased part of the lungs and reduce hyperinflation. This helps the healthier parts of the lungs to expand, lifting pressure off the diaphragm—decreasing shortness of breath, making breathing easier, and enabling patients to be more active.
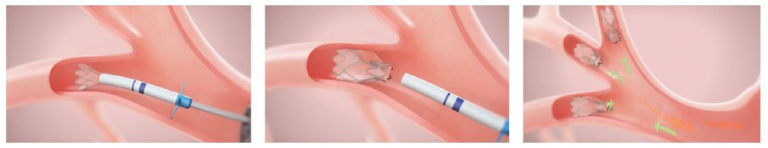
“The valves are placed via bronchoscope passed through the mouth to the lung,” Dr. Abouzgheib explains. “It’s much less invasive than traditional lung volume reduction surgery, and patients are hospitalized for only four days, just for observation.”
“The most serious potential side effect with BLVR is pneumothorax, which can happen right after the procedure, so that’s why we keep patients for that length of time,” he adds. Conversely, he notes, open surgery requires a much longer stay and the placement of chest tubes on both sides, and he notes, “sometimes patients did not do well.”
Dr. Abouzgheib also points out that the BLVR procedure is reversible. “If necessary, the valves can be removed the same way they were placed, via bronchoscope,” he says.
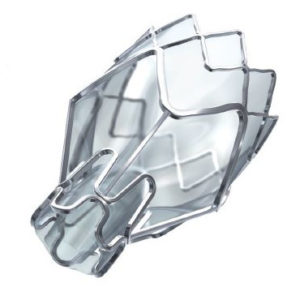
Zephyr Valve, image courtesy of Pulmonx Corp.
Two companies were granted FDA approval in 2018 to market the bronchial valves in the United States for treating severe emphysema: Pulmonx® Corp. for its Zephyr® Endobronchial Valve System and Olympus for its Spiration Valve System. Each was deemed “a breakthrough technology… that offers significant clinically meaningful advantage over the current standard of care.” Before they received FDA approval, the devices were studied in multiple randomized, controlled clinical trials, and they have been used to treat thousands of patients outside the United States.
Cooper began offering BLVR in July and was the first hospital in the state to do so. As of mid-September, Dr. Abouzgheib had performed the procedure on eight patients.
“Most are feeling significantly better when it comes to shortness of breath,” he says. “They’re able to do more activities during the day and to walk farther without having to stop. Some also are reporting they use their home oxygen less.”
“If you have a patient with advanced emphysema who wants to be more active, we encourage you to refer them for initial screening to see if they’re a candidate for BLVR,” Dr. Abouzgheib says to community physicians. “We want to partner with you to help your patients feel and breathe better.
“It’s very gratifying to be able to change a patient’s life in this way,” he adds.
For a physician to physician consultation, email Abouzgheib-Wissam@CooperHealth.edu.
